
Vanadium-rich Ruby from Mogok, Myanmar

by Dr. M. S. Krzemnicki, first published in Facette 25 (February 2019)
Mogok-type rubies are known to contain small but distinct amounts of vanadium (often in the range of 0.015 – 0.025 wt% V2O3), but are commonly highly dominated by chromium (approximately by a factor of 20-100 x) responsible for their often attractive and saturated red colour and strong fluorescence. In some cases, vanadium enriched rubies and purple sapphires from Mogok have been reported (Zaw et al., 2014 and Sutherland et al. 2015), especially from the western part of the Mogok Stone Tract that extends into a zone from Pingu Taung (Kyatpyin), Yadanar Kaday-kadar, to Singh-wa.
Recently, the SSEF received an unheated Burmese ruby (Figure 1) which showed a very uncommon and high vanadium concentration (up to 0.51 wt% V2O3) compared to rather low chromium (0.05 wt% Cr2O3) and very low Fe (0.005 wt% Fe2O3 ). This trace element composition (analysed by ED-XRF) results in a V/Cr-ratio of 11.4, which is distinctly higher than those commonly encountered in Burmese rubies (V/Cr-ratio about 0.1), but similar to reported V-concentrations and V/Cr-ratios reported by Zaw et al. 2014. The ruby showed a slightly purplish colour of strong saturation (Figure 1), especially visible in daylight equivalent light (6500 K). Using a warmer white light (about 4000 K), the colour was distinctly less purplish and much more a dark saturated red. The inclusions were very characteristic and classic for Burmese rubies from marbles, showing distinct swirl-structures (Figure 2), colourless carbonate-inclusions, and zones with dense and fine rutile needles.

Minerals (e.g. corundum, spinel, chrysoberyl, beryl) coloured by vanadium (V3+) or chromium (Cr3+) often show a very similar absorption spectrum, dominated by two absorption bands in the visible to ultraviolet range, with the chromium absorption bands located at slightly lower wavelengths than those caused by vanadium. This slight shift may however have quite an effect on the apparent colour of such materials (e.g. shift from colourchanging alexandrite to green vanadium chrysoberyl).
Vanadium in (synthetic) corundum is known to produce a greyish–purplish colour change (somehow imitating alexandrite), whereas chromium in rubies results in a pure red colour. In the case of small traces of vanadium besides the main colouring elements (e.g. Cr, Fe and Ti), the effect on the colour in rubies and sapphires can be neglected. However, higher amounts of vanadium in sapphires shifts their blue colour slightly to purplish blue hues, in some cases even resulting in a slight to moderate colour change.
The described V-rich ruby is a perfect sample to study the effect of high vanadium (and low Cr) on the colour of rubies. It shows a saturated purplish red hue which can be definitively attributed to the high vanadium concentration. In addition, this ruby shows only a very dull red fluorescence reaction in longwave ultraviolet, thus indicating that the high vanadium concentration effectively reduces the Cr-fluorescence even in the near absence of any iron in the crystal structure.
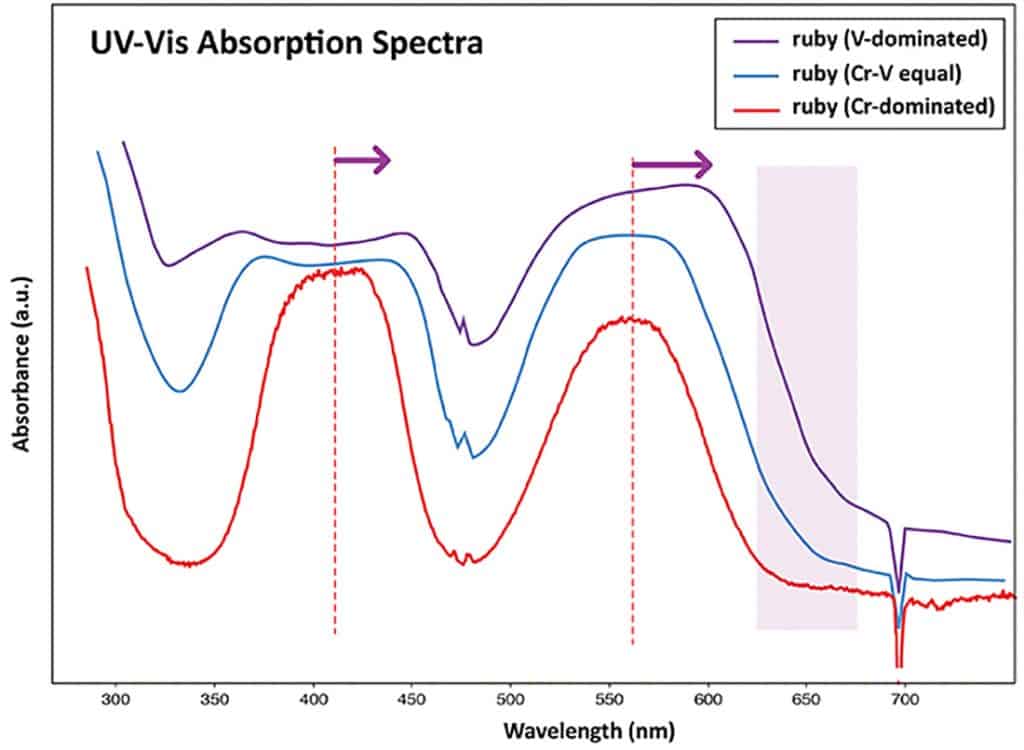
A comparison of the absorption spectra (Figure 3) of this V-rich sample with a normal Cr-dominated (and Fe poor) ruby from Mogok illustrates the above described shift of the two main absorption bands (purple arrows) towards longer wavelengths due to the high vanadium concentration (combined with a distinct reduction of transmission in the UV range). As a result, the transmission in the red spectral range is reduced in this vanadium rich ruby (light purple zone in Figure 3), which explains its colour shift towards purplish red as the transmission window in the blue gets more important. However, due to the stronger crystal field effect of Cr3+ compared to V3+, even small chromium concentrations contribute more to the colour (red) than vanadium (purple-grey addition) (Zaw et al. 2014). Consequently the identification of the described specimen as ruby is evident as its colour is still distinctly chromium-related, although chemically dominated by vanadium as a trace element.
The high vanadium concentration of this and other related rubies clearly indicates that vanadium is a major characteristic of Burmese rubies, notably from Mogok and related marble deposits (Zaw 2014, Sutherland et al. 2016), which sets them apart from other marble deposits along with the Himalayan mountain range (e.g. Tajikistan, Afghanistan, Vietnam) or in East-Africa (e.g. Tanzania, Kenya), to name a few.


