The Challenge: Identification of a Ramaura Synthetic Ruby
by Dr. Michael S. Krzemnicki, first published in Facette 22 (February 2016)

Similar to fashion trends, gemmological issues challenging gem-testing laboratories have changed over time. In the 1980s, synthetic coloured stones threatened the trade. But since the early 1990s, the challenge has switched to treatment and disclosure issues, such as flux-assisted heating (of rubies mainly), fissure-filling of emeralds, beryllium-diffusion of corundum, and rather lately low-temperature heating of corundum. As a consequence of this change, identification of synthetic gemstones produced decades ago may become occasionally very challenging especially for younger gemmologists, as such material is nowadays reappearing only sporadically and is so much out of focus of current gemmological research and teaching. The following may thus be taken as a good example to illustrate this challenging situation.
Recently, the SSEF received a ruby of 6.02 ct set in a ring for testing (Figure 1) accompanied by documents claiming it to be an unheated ruby of natural formation but of unknown geographic origin.

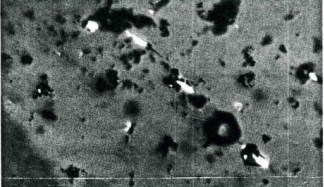
Chemical analyses (by ED-XRF) revealed a rather ‘normal’ trace element pattern, with equal amounts of chromium and iron and traces of gallium, as might be expected for example of rubies from Montepuez (Mozambique) or Winza (Tanzania). Much to our surprise, detailed microscopic investigation however showed features very similar to what can be expected in flux-melt synthetic rubies. Apart from the lack of any natural solid or fluid inclusion within this ruby, we found marked parallel zoning and numerous very fine veil-type healed fissures filled with granular-looking orange material (Figure 2). Such granular fillers are most characteristic and specific for flux-melt synthetic rubies.

To fully understand this situation, we did full analytical characterisation of this ruby by LA-ICP-MS (Laser Inductively Coupled Plasma Mass Spectrometry), FTIR, and Raman, and compared the results with flux-synthetic ruby references from our SSEF collection (and the H.A. Hänni gemstone collection).
A large surface-reaching cavity filled with a granular orange substance (Figure 3) provided clues not only to identify the material as synthetic, but also to identify that this stone had been produced by the US company J.O. Crystal, which market their product as RamauraTM synthetic rubies (http://www.ramaura.com) since more than twenty years.
By its visual appearance, this orange foreign material could also be misinterpreted as naturally occurring iron hydroxide. However, LA-ICP-MS trace element analyses on the orange material in the cavity revealed that it is highly enriched in exotic elements such as boron, lead, bismuth and lanthanum, characteristic and in fact required components of the flux in which Ramaura synthetic rubies grow (Kane 1983, Schmetzer 1986, Muhlmeister et al. 1998). Comparing our data with Ramaura synthetic rubies from the SSEF collection showed that they were highly matching not only in their trace element concentration, but also inclusion features and Raman spectrum of the granular orange flux residue.
What made this synthetic ruby an intriguing case is its rather ‘natural looking’ chemical composition as detected by ED-XRF, with equal amounts of chromium and iron and traces of gallium. However, the absence of any titanium and vanadium in the investigated ruby (and in the Ramaura synthetic reference, for both confirmed by LA-ICP-MS) should make a gemmologist cautious, as these two elements are usually present in any natural ruby at trace levels due to geochemical reasons.
Although the inclusions are quite characteristic of its synthetic formation for an experienced gemmologist, they may be misinterpreted (and obviously had been so, before SSEF tested the specimen) as naturally occurring iron-hydroxide residues due to the fact that gem labs (and the trade) are nowadays only rarely exposed to such challenging synthetic samples.
Want to learn more about rubies?

Sign up for our free online course: Introduction to rubies