
Beware of synthetic ruby with ‘zircon-like’ cluster inclusions
by Dr. M.S. Krzemnicki, first published in Facette 27 (June 2021)

Synthetic stones are rather rare guests at SSEF, as most of them are sorted out already before submission to SSEF based on their tell-tale characteristics. Those which finally come to us are therefore rather uncommon or even tricky cases (see e.g. SSEF Facettes No. 22, 2016, page 14; and No. 25, 2019, pages 12-13), which may even have fooled other gemmologists and gem labs alike.
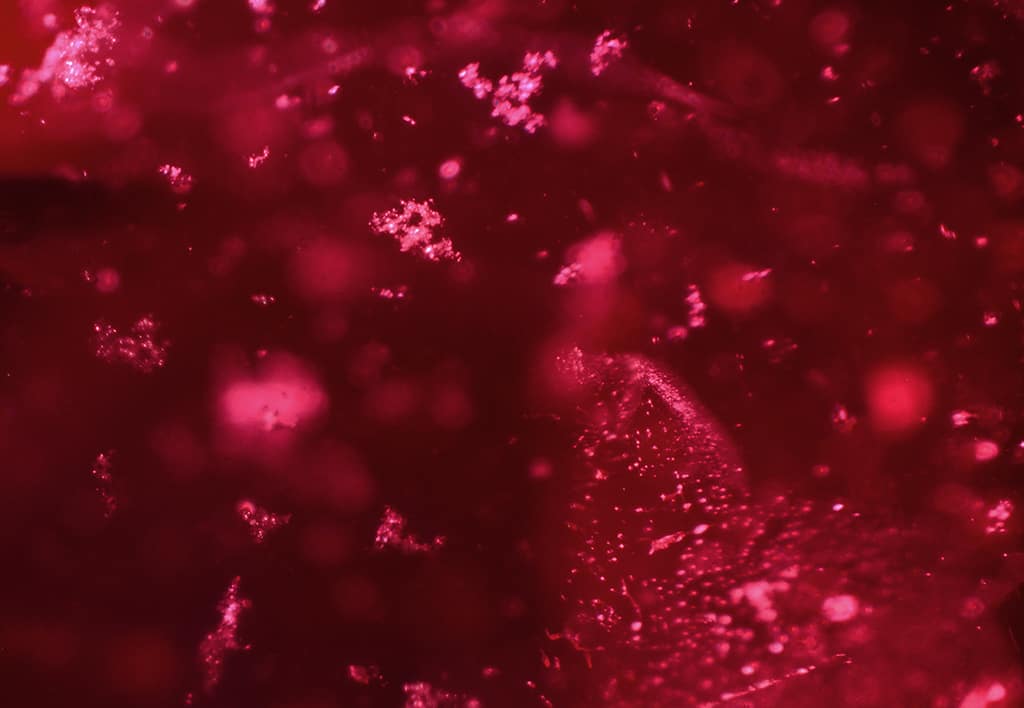
Very recently, we tested a tricky case of a synthetic ruby (Figure 1) of 4.27 ct, submitted to SSEF as a natural stone accompanied by two lab reports confirming its natural origin. Under the microscope, the submitted stone showed ‘zircon-like’ cluster inclusions (Figure 2). They were somehow reminiscent of zircon clusters commonly observed in rubies from Vatomandry in Madagascar.
Raman spectroscopic analyses, however, revealed that these clusters are in fact made of an unknown solid substance. Their Raman spectra were not matching with zircon, xenotime or any other natural inclusion commonly found in corundum. Additionally, the stone contained no further natural inclusion, such as rutile, monazite, apatite, or amphibole, as could be expected in a natural ruby of this size, specifically from Vatomandry. In addition, this stone showed a very strong red UV-reaction (typical for iron-poor rubies), which is in contradiction to rubies from Vatomandry that usually show a dull red UV reaction due to their distinct iron concentration.
Detailed chemical analyses using both EDXRF and LAICPMS clearly excluded a natural origin for this stone. This conclusion could be drawn mainly due to the presence of exotic trace elements, actually related to the flux-melt synthesis but not to any geological process. These exotic elements included amongst others: manganese, antimony, rhodium, zirconium, and platinum.
Another interesting aspect was the complete absence of vanadium in this synthetic ruby (detection limit with LAICPMS is below 0.06 ppm!). Every natural ruby contains some traces of vanadium, as this element is geochemically linked to chromium, which acts as colouring element (chromophore) in rubies.
Similar synthetic rubies are known since quite some years (e.g. Atichat et al., 2012, GIT Conference Proceedings). Due to their ‘zircon-like’ clusters, they are rather difficult to recognise and may even fool an experienced gem trader and gemmologist.
Want to learn more about rubies?

Sign up for our free online course: Introduction to rubies


