Rarities and Collector Stones Tested at SSEF

The daily routine at SSEF usually consists of testing rubies, sapphires, emeralds, pearls and diamonds. Although sometimes challenging and interesting, especially taking into account the quality and the documented historical provenance of some of these gems and jewellery items, it is also always of great interest and scientific value for us to analyse rare minerals and collectors stones, which are occasionally sent to SSEF. In the past few months, we have been especially fortunate to analyse and document an eclectic mix of such gems, a few of them that will be presented in the following.
A rare collection of pezzottaite
Pezzottaite is a rare and attractive pink mineral which was discovered only in 2002 in a few pegmatites in Madagascar, Afghanistan and lately the Mogok Stone Tract in Myanmar. This mineral bears a close structural relationship to beryl, however it shows a trigonal crystal structure due to the ordered substitution of part of beryllium by lithium, coupled with caesium in its channel structure (Hänni & Krzemnicki 2003 & 2004, Laurs et al. 2003). Named after Dr. Federico Pezzotta, it immediately drew the attraction of gemstone collectors due to its attractive pink colour, sometimes additionally highlighted by a distinct cat’s-eye effect.
Recently, the SSEF had the opportunity to investigate an impressive collection of more than 20 faceted pezzottaites ranging in size from 100 ct to 6 ct (Figure 1). The gemstones were readily identified based on their elevated Cs concentration and characteristic Raman spectrum (Hänni & Krzemnicki 2003; Lambruschi et al. 2014). Having analysed a number of rather small specimens just after the discovery of this new mineral in 2003, we were stunned to see a collection of such large faceted pezzottaites. Due to their outstanding size, they perfectly displayed their distinct pleochroism when observed in different orientations (Figure 2). Not to our surprise, a detailed study with FTIR and Raman revealed that the fissures present in these pezzottaites were filled with colourless artificial resin and oil to enhance their clarity.

Rare twins: Musgravite & Taaffeite
Having analysed a number of rather tiny musgravite and taaffeite samples from East Africa a few years ago for a research study (Schmetzer et al. 2007), we were stunned by three exceptional speci- mens which were submitted to us over the last few months for testing.
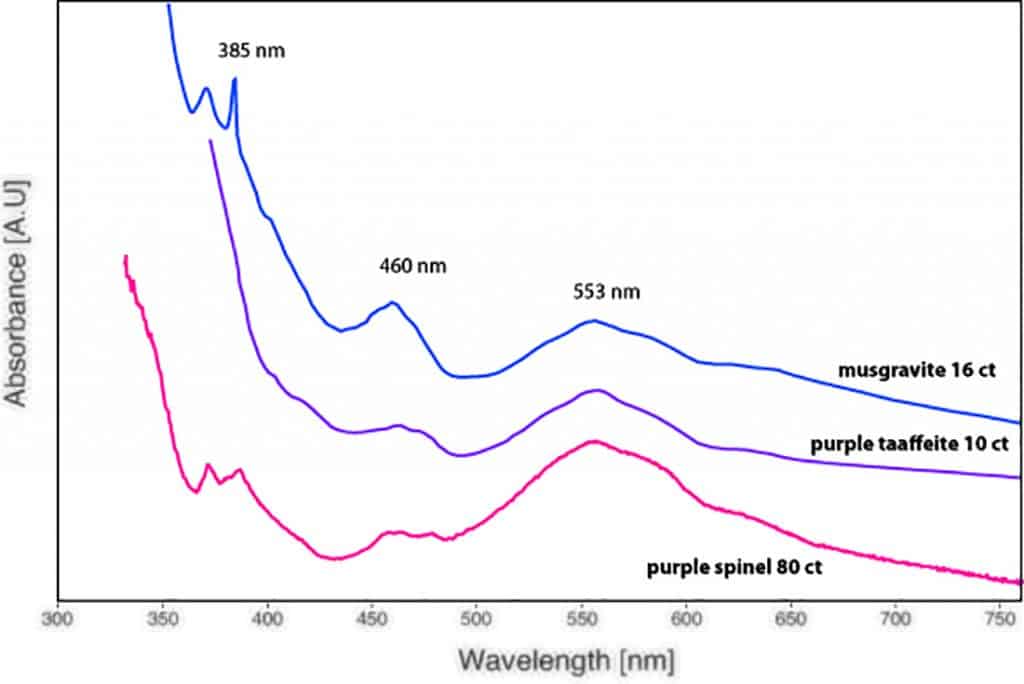
The first of these three stones was a heart-shaped musgravite of 16 ct, a size very impressive when compared to the normal range of 0.5 – 2.5 ct for this very rare collector mineral and gemstone. A second musgravite of 11 ct was submitted later in 2016 by another client, again of outstanding purity and quality. And finally, a further client submitted an exceptional taaffeite of 10 ct of purple colour (Figure 3) and very fine purity, except for a few tiny hollow channels filled with brownish orange iron hydroxide (Figure 4).
Gem-quality musgravite and taaffeite visually show a close resemblance to grey to violet spinel, but are optically anisotropic compared to isotropic spinel. They typically are very small and range in colour from purple to grey and greenish grey, with purple being the most appreciated colour. They have been found in few and mostly small specimens in Sri Lanka, Tanzania, Madagascar, and Mogok (Myanmar), which are famous for their wealth in gemstones.
Taaffeite, ideally BeMg3Al8O16, is a very rare collector mineral named after Mr Richard Taaffe, who by chance discovered the first specimen in 1945 in a jewellery shop in Dublin (Ireland). Due to its visual appearance, the specimen was offered to him as a spinel and was described as a new mineral species only after his lucky discovery. Musgravite, ideally BeMg2Al6O12, was first discovered in 1967 in rocks of the Musgrave Range (hence its name) in central Australia.


Taaffeite and musgravite are closely related in chemistry and structure and can only be separated using sophisticated structural analysis, such as Raman spectroscopy. Although renamed to magnesiotaaffeite- 2N’2S (taaffeite) and magnesiotaaffeite-6N’3S (musgravite) by the International Mineralogical Association (IMA) due to their structural polytypism, the original names taaffeite and musgravite are still commonly used in the gem trade and evoke appreciation by collectors worldwide.
Interestingly, the (light) purple taaffeite showed an absorption spectrum mainly dominated by Fe-bands and no chromium band centred at 562 nm (Schmetzer at al. 2000). As such, the absorption spectrum looked similar to (slightly purplish) grey musgravite and purplish grey spinel (Figure 5). The photoluminescence spectrum (using a 514 nm laser excitation) of the same sample revealed only tiny Cr luminescence bands (doublet at 684.6 nm & 685.7 nm) and no chromium concentration in the chemical EDXRF analysis. Based on these results, we can conclude that the purple colour in our studied sample is mainly due to iron and not linked to the presence of chromium.
Star Spessartine from East Africa
Spessartine, the manganese end member of the garnet group is characterised by a vivid orange colour, which makes them very attractive gemstones known also as Mandarin garnet in the trade. In terms of a rare specimen, we recently had the pleasure of analysing a spessartine garnet originating from East Africa that was characterised by a distinct six-rayed star effect. Asterism in garnets has been known and documented since decades (see Schmetzer et al. 2002 and references therein). However, all available literature so far describes asterism in dark purplish brown almandine to purplish red pyrope, making this star spessartine an interesting sample.
The studied sample is a cabochon of 27 ct, displaying an even and well centred six-rayed star (Figure 6). EDXRF analysis confirmed its identity as a spessartine garnet, very much dominated by manganese on the dodecahedral {X3} structure with some magnesium and only low iron concentration. A detailed microscopic and Raman microspectrometric study revealed a dense and complex pattern of aggregated clusters (Figure 7) consisting of intersecting oriented rutile needles accompanied by flat void platelets, partly encrusted with tiny colourless feldspar grains. Raman microspectrometric analyses confirmed the needles to be rutile, encrusted with tiny feldspar grains. The star effect visible in this specimen is mainly due to the rutile needles, possibly aligned along [110] crystallographic directions, which form, when viewed perpendicular to the octahedral face [111] three sets of needles intersecting at 60° to each other (Schmetzer et al. 2002), and thus a beautiful and regular six-rayed star after having been cut appropriately as a cabochon.


Excellent Example of imperial topaz
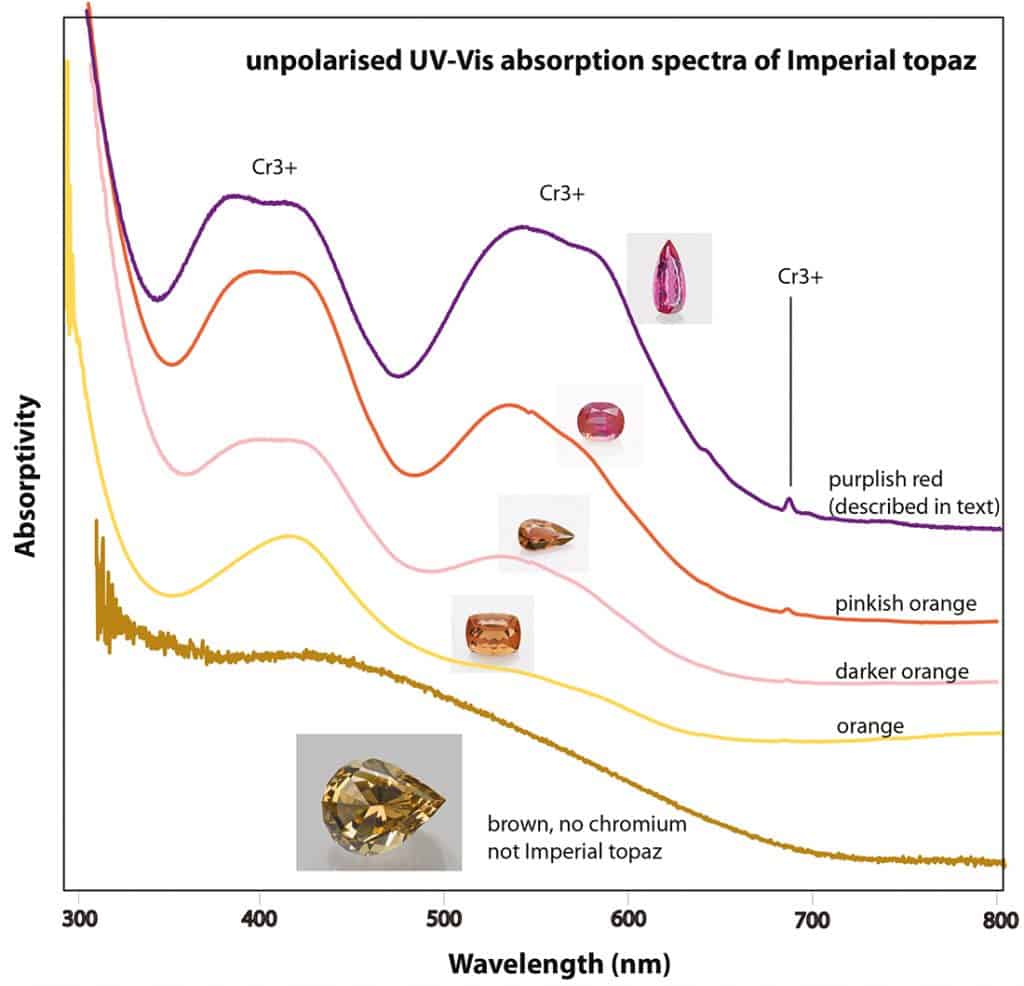
Topaz is found in many colours, with the pinkish to orange colours being the most sought after and highly priced varieties. Especially topaz in this colour range from Ouro Preto in Minas Gerais (Brazil) has a high reputation and is often named ‘imperial topaz’ in the trade. Although there is no harmonised criteria for this trade term – but inline with most literature sources – we consider topaz of pink to orange colour with distinct chromium absorption bands (and thus reddish fluorescence reaction under longwave ultraviolet illumination) as ‘imperial topaz’ and mention this trade term in the comments section of our SSEF reports.
The pear-shaped topaz of 32 ct specimen described in the following is less a matter of scientific research or curiosity, but more an example of pure beauty, which we had the pleasure of analysing at SSEF. Its analysed properties are consistent with those of topaz from the classical mining sites near Ouro Preto in Brazil. The described topaz is characterised by a saturated purplish red colour accentuated by attractive vivid orange red hues at the base and tip of the pear shape, thus perfectly illustrating the influence of the cutting style on the colour appearance of this topaz (Figure 8). Its colour is due to distinct amounts of chromium 0.17 wt% Cr2O3) in combination with so-called structural colour centres responsible for the orange red hues (Figure 9). A heat treatment can be excluded, as such heating would result in a deactivation of those colour centres and thus a shift of colour to pure purplish red.

Colourful Tourmalines
When it comes to tourmaline, the chemical element copper is kind of the ‘magic’ ingredient in terms of colour and finally market appreciation. In the past few months, we have had the chance to analyse two very specific tourmalines especially in regards to their copper concentration.

Figure 10: Copper bearing tourmaline (40 ct) of vivid purple colour from Mozambique and tourmaline of weak greenish blue saturation containing only small traces of copper, and thus not considered Paraiba tourmaline by SSEF. Photo: SSEF
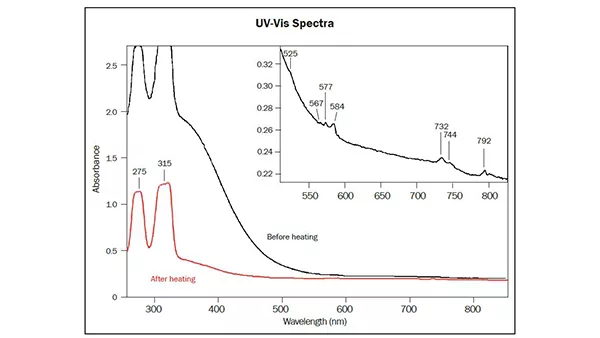
The first specimen was an impressive copper-bearing tourmaline (40 ct) of vibrant and vivid purple colour and exceptional purity (Figure 10) originating from Mozambique (East Africa). The chemical analysis revealed minor amounts of copper and manganese as main contributors for the attractive colour of this gem, and iron below detection limits. The UV-Vis absorption spectrum showed two characteristic broad Cu-bands in the near infrared (about 700 and 920 nm) and a high and dominant Mn3+ absorption band (centred at 530 nm), which in fact is responsible for the vibrant colour due to the marked transmission windows on the sides of this absorption band (Figure 11). It is a well known fact that this manganese absorption band quickly disappears when heating such tourmalines at rather moderate temperatures (Abduriyim et al. 2006; Milisenda et al. 2006; Laurs et al. 2008). Consequently, the colour of such Cu-bearing purple tourmalines will shift into a highly desired and more expensive vibrant blue colour through the heating process. This explains why most copper-bearing purple tourmalines are heated. In this case, it was a stunning experience to see how beautiful the purple colour of such copper-bearing tourmalines can be if spared from heat treatment.
The second tourmaline was of even larger size (60 ct), showing a subtle greenish blue colour of weak saturation (Figure 11). The chemical analyses of this tourmaline revealed a very low concentration of copper (0.04 wt% CuO), together with manganese and iron as minor constituents. Even though of very low concentration, the copper was still partially responsible for the colour, in combination with iron (Fe2+ – Fe3+ intervalence charge transfer absorption band at about 720 nm). Although containing traces of copper, this tourmaline of weak colour saturation is not considered a Paraiba tourmaline, based on our SSEF standards and LMHC guidelines (LMHC Information Sheet 6 on Paraiba tourmaline), but still an extraordinary gemstone of subtle and appealing colour.
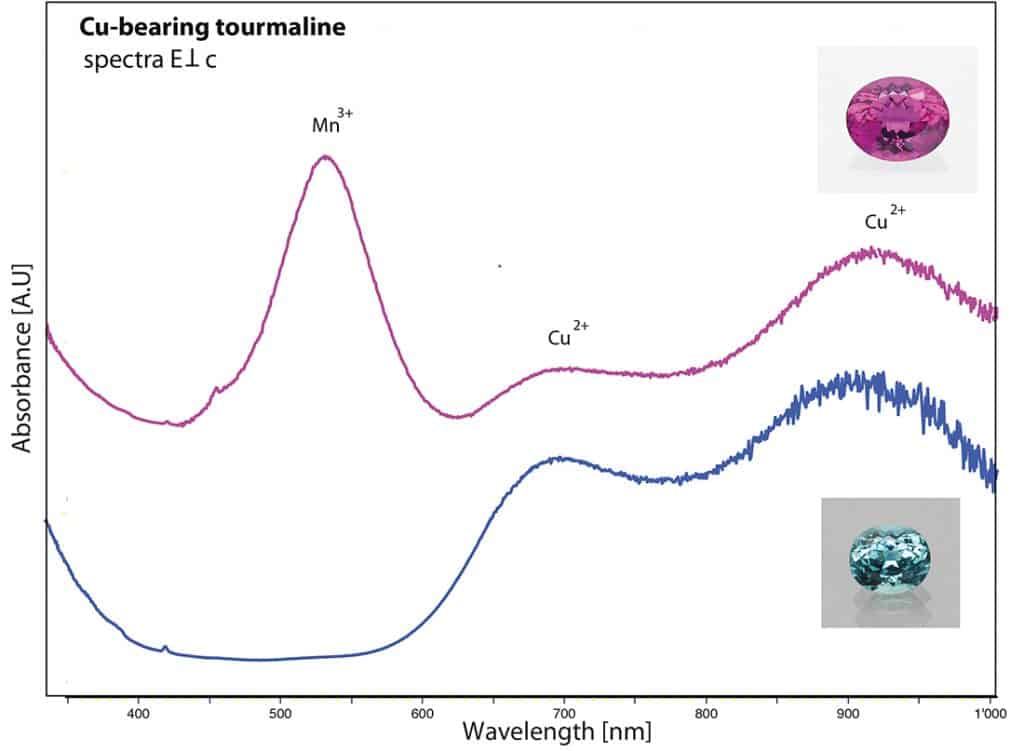
Figure 11: Absorption spectrum of the described purple tourmaline (40 ct), showing very distinct Cu and Mn absorption bands which are responsible for its vivid colour, compared to the spectrum of a heated blue copper-bearing (Paraiba) tourmaline from Mozambique. The absorption band at 530 nm related to Mn3+ disappears during heat treatment, resulting in a shift of colour from purple (before heating) to blue (after heating). Figure: M.S. Krzemnicki, SSEF