To be, or not to be, that is the question: chrysoberyl versus alexandrite
by Dr. M.S. Krzemnicki, first published in Facette 27 (June 2021)

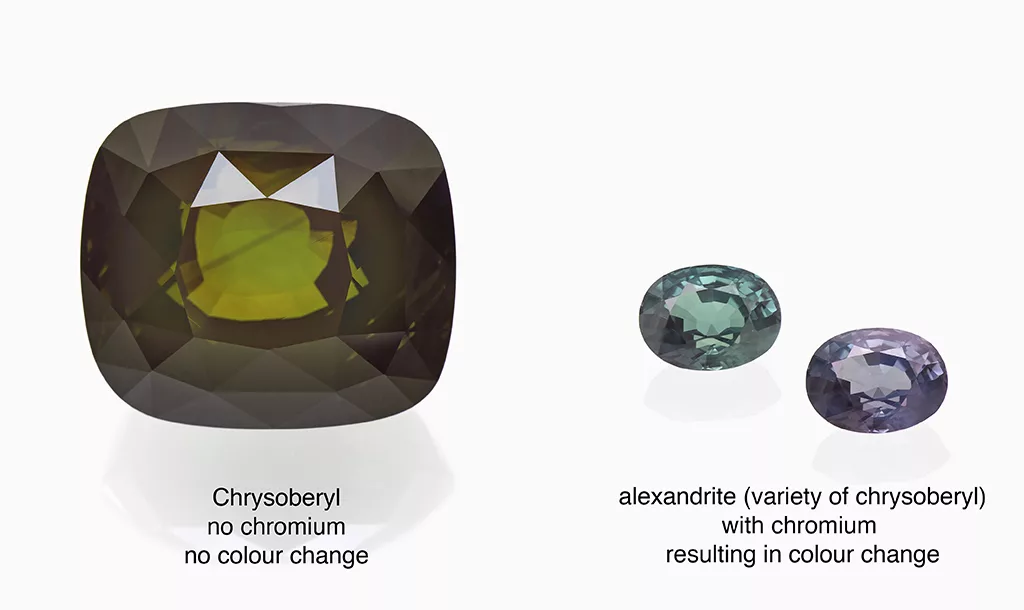
The mineral chrysoberyl BeAl₂O₄ is a highly appreciated gemstone due to its rarity, brilliance, and beauty and comes in attractive colours commonly ranging from colourless (chemically pure) to yellow, yellowish green, green, and brownish green to dark brown, mostly related to the presence of iron in its crystal structure. In case of the presence of chromium, this mineral will show a colour-change commonly from green or bluish green in daylight to purple to reddish hues in incandescent light (e.g. tungsten lamp). In that case, the gem is called alexandrite, a sought-after variety of chrysoberyl. This chromium-related definition of alexandrite is internationally recognised and dates back to the early scientific description of this attractive gemstone first discovered in the Ural mountains in Russia in the mid-19th century.
At SSEF, we receive from time to time chrysoberyl specimens for testing which do not contain any chromium (or only very low traces), but which are mislabelled as alexandrite. Such was the case with a very large and impressive brownish green chrysoberyl we received recently at SSEF (Figure 1). This stone contained only about 0.002 wt% chromium, but about 600 times more iron (about 1.2 wt% Fe₂O₃), so in no way could qualify as alexandrite.
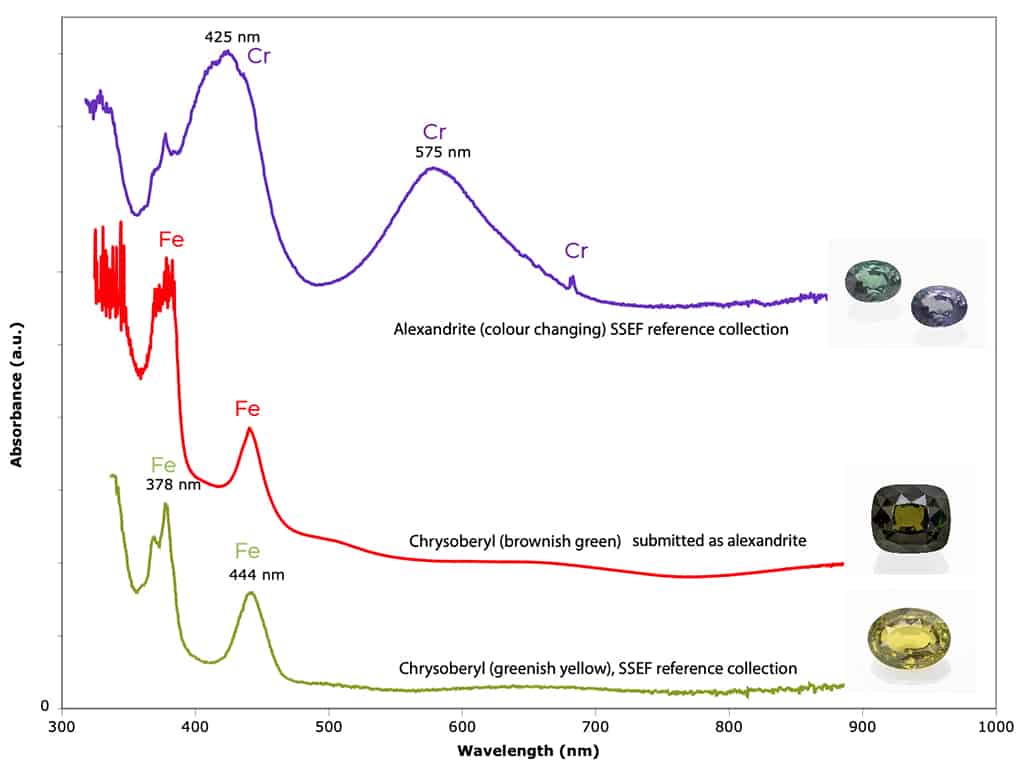
To set the record straight: to be called alexandrite, a chrysoberyl has to fulfil three basic criteria: 1) It has to show a moderate to distinct colour change observed under standard lighting situations (daylight and incandescent light). 2) It has to contain a distinct amount of chromium and 3) as a consequence has to show a distinct absorption band at about 575 nm (related to Cr3+) (see Figure 2). Although greenish brown to yellowish brown chrysoberyl may show a slight colour shift towards a slightly more brownish hue in incandescent light, such stones do not qualify as alexandrite, as their colour is mainly or completely related to iron.
We at SSEF consider it very important that gemstone variety names are carefully and correctly applied based on internationally accepted nomenclature. We are confident that this is in the best interest of the gem trade and finally the consumer who wants to trustfully buy a valuable gemstone.
For interested readers: More about the application of variety names at SSEF is found in the last Facette, issue 26 (2020) and in a SSEF presentation (2019) about gemstone varieties (see https://www.ssef.ch/presentations/).


