Diamonds as a window into the earth
by Dr. L. Speich, first published in Facette 26 (May 2020)
Diamond is valuable as a gemstone but it is also a mantle geologist’s best friend because it provides a rare opportunity to study processes that occur deep in the Earth. Most diamonds form in the so-called lithospheric mantle within a fairly narrow depth-range between 140 and 200 km. However, diamonds from deeper sources, some in excess of 600 km, have been reported as well and continue to fascinate geologists.
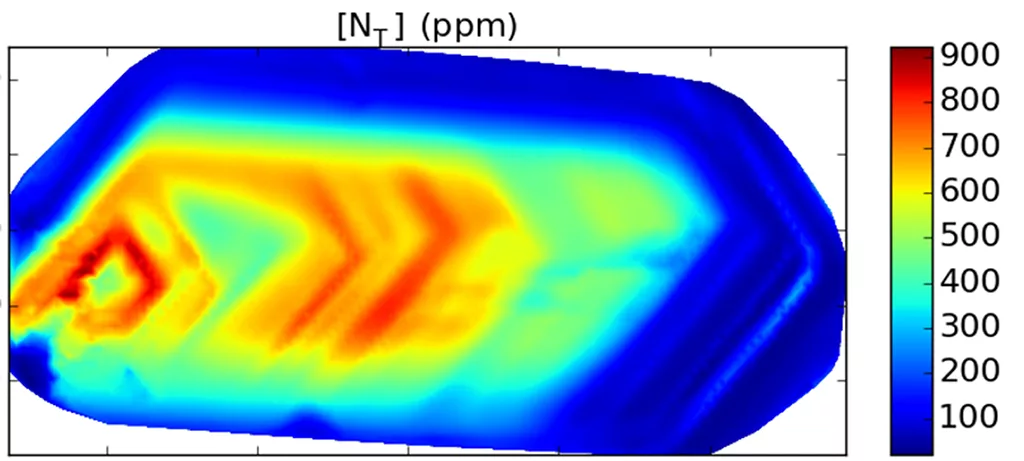
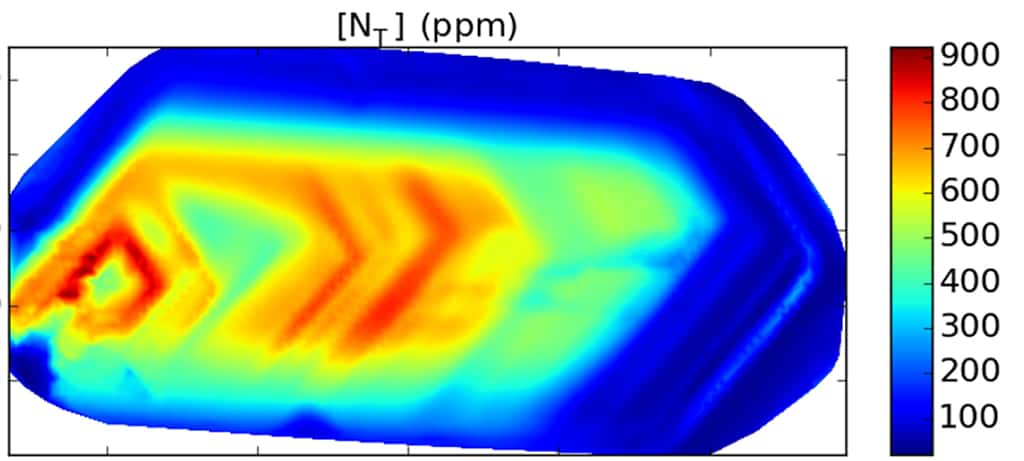
Diamond is a valuable source of information for geologists because they can contain mineral inclusions, small pieces of other minerals present in the mantle entrapped within the diamond during growth. Protected by the diamond almost like a time-capsule, these are the only pristine samples of mantle material that geologists can study. But inclusions are not the only aspect about diamonds that make them valuable for research. Diamonds can contain a number of impurities such as nitrogen and hydrogen that can be present in different configurations in the crystal lattice. Some of these defects, most notably those related to nitrogen are known to transform according to the conditions the diamond has experienced. As a result, geologists can reconstruct the history of a diamond, and thus study conditions and processes that occur at depths that are otherwise inaccessible. The same circumstance allows gemmologists to accurately identify synthetic and heat-treated diamonds because they have been subjected to conditions that are different to naturally grown untreated stones. Thus, understanding the formation of diamond in the mantle, their storage and journey to the surface and the traces these processes leave is important in gemmology as well.
Nitrogen is the most common impurity in natural diamond. At mantle conditions (diamonds form at pressures on the order of 5-7 GPa and temperatures of 1100-1250˚C), nitrogen is mobile and can move through the crystal lattice to form different types of defects. This process referred to as nitrogen aggregation takes several hundred million or even billions of years to complete. When the diamond is brought to the surface by kimberlite magma, the process is stopped in its tracks. Using infrared spectroscopy, its progress can be measured and the temperature of mantle residence can be calculated.
Want to learn more about diamonds?

Sign up for our free online course: Introduction to diamonds


