
Colour varieties of gems – where to set the boundary?
by Dr. M.S. Krzemnicki, first published in Facette 26 (May 2020)

In theory it is simple: a gemstone is a mineral formed in nature by geological processes and, as such, it has a mineralogical name that is scientifically defined and accepted by the International Mineralogical Association (IMA) and its Commission of New Minerals, Nomenclature and Classification (CNMNC). In some cases, this mineral name is known and valued by the trade and consumers (e.g. diamond) and does not need further classification. However, for most coloured gemstones, things are much more complex, as most of them are known to consumers and the trade only by their variety names.
Generally, variety names are related to variations in chemical composition and colour of a mineral. Some variety names are well-known in literature since the advent of modern mineralogy in the 18th century (e.g. ruby, sapphire, emerald), whereas others have been introduced in the last few decades with the aim of making a new gem material more appealing in the market (e.g. tanzanite for vanadium-bearing variety of zoisite, tsavorite for vanadium-bearing variety of grossular garnet). In some cases, such variety names are also linked to external appearance, such as for example single crystalline quartz (e.g. rock crystal) and polycrystalline chalcedony. Although the classification of variety names often seems straightforward (e.g. emerald for the chromium-bearing green variety of beryl and aquamarine for the iron-bearing light blue variety of beryl), we need to remember that they are generally rather vaguely defined, especially when it comes down to separating different varieties of the same mineral from each other (Hughes 1994).
In this article, we would like to provide insight into the issue of classifying coloured gems into their respective varieties and present a number of case studies to illustrate the topic from a laboratory perspective. This is based on an earlier presentation given on the topic and that also provides further examples (see Krzemnicki, 2019 under www.ssef.ch/presentations).
Creation of Standards
The main prerequisite for any gemmological laboratory is to follow an internally defined standard procedure to be able to evaluate and classify colour varieties of gems in a consistent manner over many years. In the absence of globally agreed standards, it may thus be necessary for a lab to create internal standards using for example selected master stones, colour measurements (based on spectroscopy), or colour tables (e.g. Munsell colour chart or ColorCodex™; Smith, 2020). Such an internal standard may later become internationally harmonized and accepted by laboratory and trade organizations (e.g. LMHC, CIBJO, ICA).
Colour observation of coloured gemstones is a complex issue, based on three main factors:
- the light source (emission characteristics),
- the observer (protocol, tools and training),
- and the observed item (e.g. ruby or pink sapphire).
To grade colour consistently, it is mandatory to use standardized light with a high colour
rendering performance (Krzemnicki, 2019). In addition, it is advisable to slightly tilt the gemstone
in all directions by 10°-20°
when observing its colour from atop (at about 25 cm distance
to light and observer)
to better judge the full colour sparkle or less desirable colour zoning
effects of a stone.

Three Case Studies
The following case studies are separated into cases where the classification of varieties is based on (1) colour and (2) colour and spectroscopy/chemistry. All these examples are based on the authors’ laboratory experiences and procedures encountered on a daily basis while testing gemstones.
Ruby Versus Pink Sapphire
Corundum coloured by traces of chromium can yield a wide range of red saturation, ranging from dark red to vivid red and light red (e.g. pink) (Figure 3). They are classified traditionally by the trade into the two varieties, ruby and pink sapphires. The boundary between these two varieties has never been internationally well defined, although it is of major importance for the trade, as there is commonly an important price difference between these two varieties.
Although it may seem obvious that a threshold value of chromium concentration to classify these stones either as ruby or pink sapphire could be defined, this hypothetical option is not applicable in reality. The reason is that such chemical analyses (usually measured on the table facet) may be strongly influenced by chemical zoning and the effects of the cutting style and proportions, thus leading to inconsistencies when using this simplistic approach. Not to speak of critical differences in chromium concentration measurements due to different analytical setups used in different laboratories.

A much more realistic approach is to separate rubies from pink sapphires based only on colour by visual comparison with colour charts or master stones. At the Swiss Gemmological Institute SSEF, we have used, for many decades, a set of synthetic corundum master stones, originally put together by ICA in the 1980s (see Figure 4). Such a master set allows the most straightforward colour evaluation, as these master stones show matching reflection patterns and pleochroic colour effects that are also present in rubies or pink sapphires being tested in the lab. Another option is to use colour charts made with corrugated metalic foils (e.g. Color Codex™, see Smith, 2020), which to some extent mimic the reflection effects due to the facets of a cut stone.
Cobalt-Blue Spinel Versus Blue Spinel
Spinel of blue colour is a highly attractive and appreciated gemstone in the trade. The blue colour may be due to traces of cobalt or iron or a combination of both elements and as a result they come in a range of colours from vivid cobalt blue to greenish grayish blue and purplish blue (Figure 5). The ‘magic’ term in this respect is cobalt, and so the key question from the trade is often whether a blue spinel is a cobalt spinel or just a more common blue spinel. This can only be solved by combining the colour observation with a very detailed analysis of its absorption spectrum. The absorption spectrum can show us how specific colouring elements (e.g. cobalt) contribute (absorption and transmission bands) to the colour of a stone, which ultimately results in the colour of the stone that we see.
Similar to the ruby/pink sapphire classification, a cobalt concentration threshold value is not applicable. The reason is that some spinels get their blue colouration from iron absorption features and may, in addition to iron, also have cobalt, in some cases even at higher concentrations than some vivid cobalt-blue spinels. The key defining characteristic is which colouring element is dominating in the absorption spectrum (Shigley & Stockton 1984; Chauviré et al. 2015; D’Ippolito et al. 2015).

Interestingly, those blue spinels that are coloured by a combination of both cobalt and iron may show a subtle and attractive colour change from purplish blue in incandescent light to blue in daylight (Senoble 2010; Hanser 2013).
Emerald Versus Green Beryl
Commonly, an emerald is described as a chromium-bearing variety of beryl, although other transition metals such as vanadium and iron may considerably contribute to their apparent green colour. Emeralds have been treasured since historic times for their saturated green colour and rarity, although emeralds of lighter saturations may occur. Emeralds are often found as rather small and included rough crystals; this is related to their complex geological formation in the context of metamorphic processes and late stage rock deformations (Giuliani et al. 2019).
In recent years, we have repeatedly seen light green to bluish green gems in the lab, mostly of exceptional size (100 carats and above) and purity (Lind et al. 1986; Hänni 1992). Many of these stones formed in pegmatites (e.g. in Nigeria and Madagascar) in a very different geological setting compared to all classic emerald deposits (e.g. Colombia, Afghanistan, Zambia, Russia, Pakistan, to name a few) (Figure 6).

In addition, the absorption spectra of many of these gems is strongly dominated by an iron-related absorption band centered in the near infrared (actually responsible for the light blue colour in aquamarine), with only very tiny chromium-related absorption bands in the visible part of the spectrum, thus slightly shifting their colour to a light green or greenish blue colour (Cevallos et al. 2012). This is very much in contrast to the absorption spectra of emeralds from the above-mentioned classic sources that are dominated by chromium (and vanadium) absorption bands with none to only a moderate iron band in the near infrared.
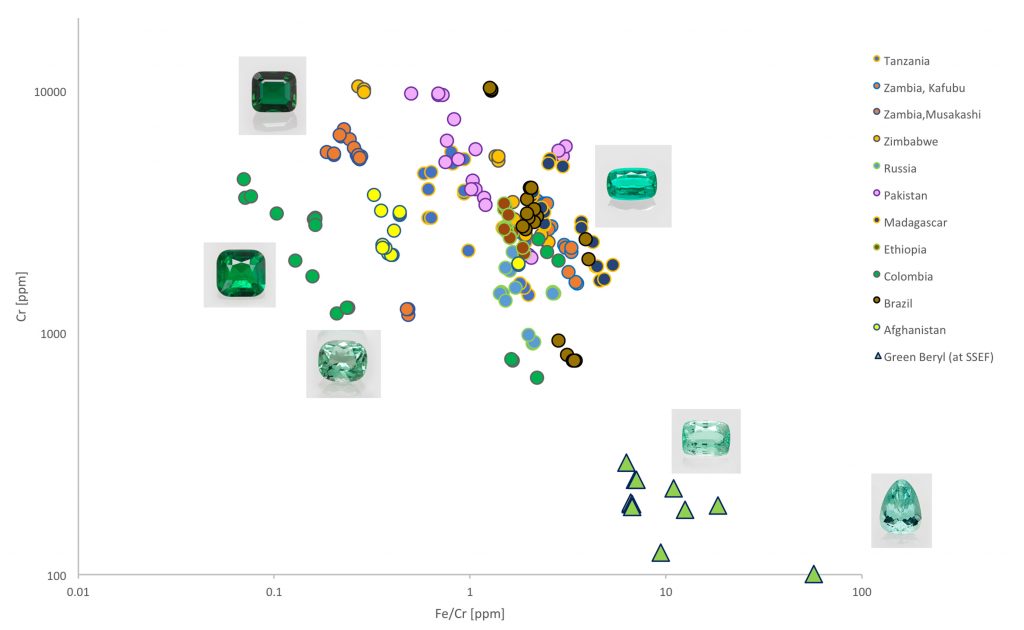
Figure 7: Plot visualizing the chemical separation of emeralds from the described light green to bluish green beryls. Figure: M.S. Krzemnicki, SSEF.
This spectroscopic difference is also well reproduced when comparing the chemical composition (chromium to iron ratio) of these two different beryl varieties (see Figure 7). The plot clearly shows two different distribution clouds, with emeralds from all the above-mentioned classic emerald sources being clearly enriched or dominated by chromium. In contrast to this, many of the light green to bluish green beryls are characterized by low chromium concentrations, but relatively high to very high concentrations of iron (about 10x-100x more iron than chromium).
Conclusions
This article sought to provide an overview of a lab’s perspective on defining colour terms using specific examples. As labs and the industry strive to achieve greater harmonization in the definition and use of variety and colour terms, it is important to be aware of the scientific challenges and limitations in doing so. As past discussions at CIBJO, GILC/ICA and LMHC have shown, there is a need to accept the complexity in defining set boundaries and the fact that ultimately a colour or variety opinion of a lab is based on observational and analytical data that ultimately forms an expert opinion. SSEF has for many years been at the forefront of these discussions within different industry forums (e.g. CIBJO, LMHC) with the aim of harmonizing the criteria and standards used by labs and the trade, and also by publishing and sharing research on these important topics. The recent CIBJO congress special reports of both the Gemmological Commission and the Gemstone Commission show that these themes are important on the industry’s agenda. We strongly welcome the trend for further harmonization and argue that it is a multi-pronged approach. This includes harmonizing definitions and harmonizing testing procedures where possible, in order to provide the trade and end-consumers greater clarity and transparency in the use of names and terms.



