An interesting chrysoberyl-alexandrite combination
By P. Lefèvre, first published in Facette 28 (May 2023)
The mineral chrysoberyl BeAl2O4 is an attractive gemstone that shows a wide variation of colours mostly ranging from yellow to green and brownish green to dark brown. These colours are mainly due to the presence of iron and sometimes vanadium as chromophore elements inside the crystal lattice structure. In some rare cases, chromium is present and has a peculiar and interesting effect as it induces a colour change of a certain intensity (weak, moderate, distinct, or strong) when the gemstone is observed under daylight or incandescent lighting conditions. Such a variety of chrysoberyl is named “alexandrite” and was first discovered in the Ural Mountains in Russia. The common observed colours of the gemstone are usually green to bluish green when observed in daylight, and switch to purple and red colours in incandescent light. This term comes from the name of the Russian Tsar Alexander II after whom the gem was named.
The orientation of a rough crystal before cutting is definitely important for any anisotropic mineral, such as corundum and chrysoberyl, as this will strongly influence the final colour of the gemstone. This will influence some optical effects as well, like chatoyancy (cat’s-eye effect) and asterism (star effect), and in some specific cases the colour change effect.
As an illustration of this last point, we recently observed at SSEF a very interesting case of alexandrite showing a moderate colour-change from green in daylight to greenish purple in incandescent light (Figure 1). As mentioned before, the cause of the colour change is due to the presence of chromium in the structure of the crystal. So we were quite astonished when the chemical analyses performed routinely on the table of the gemstone were not detecting chromium, but only iron. To understand more the causes of the colour, an absorption spectrum was performed and this additional data showed that chromium was present inside the stone in a sufficient amount to induce a colour change. So, where is the chromium located in this gemstone?

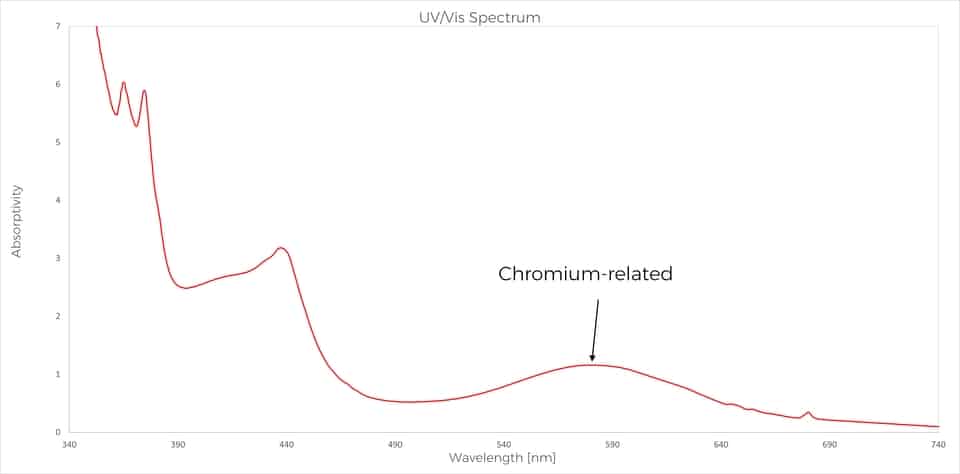
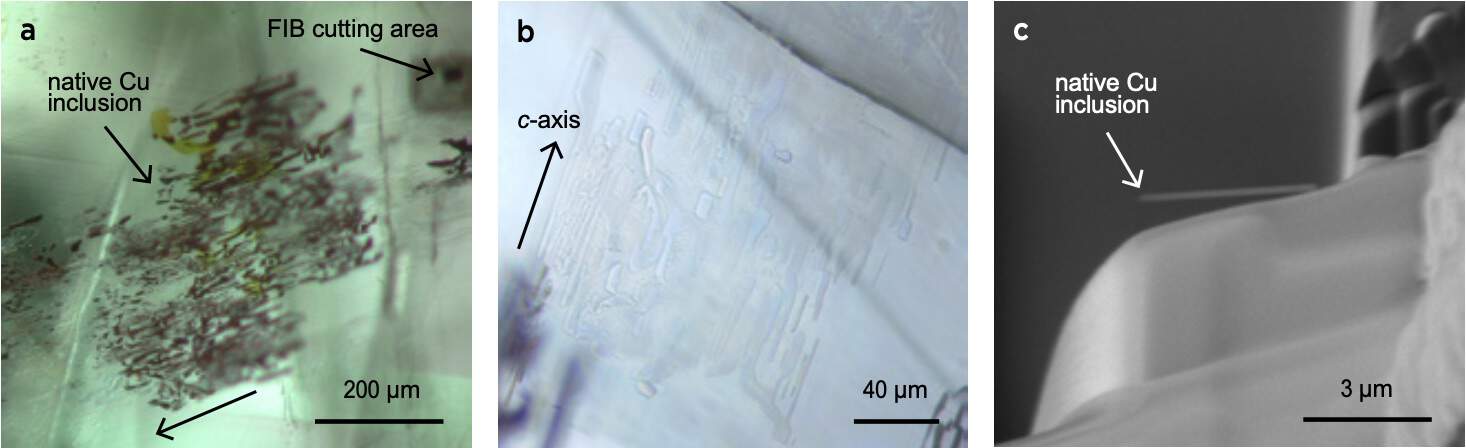
The microscopic observations revealed some common inclusions for a chrysoberyl: healing fissures, milkyness, and a distinct growth zonation (Weeramongkhonlert, 2020). The specificity of this growth zonation was that the area located at the culet on the pavilion was showing a red colour when using a strong optic fibre light (Figure 3). New chemical analyses performed this time specifically on the culet side finally revealed a significant amount of chromium with almost 0,5 wt.% of Cr2O3, which is quite a high amount for an alexandrite.

The importance of the orientation of the rough crystal before cutting and the ability of the cutter show here all their importance. If the crystal would not have been properly oriented to get the chromium-rich growth zone at the culet of the final cut gemstone, the colour-change effect would have been strongly reduced, or even not existing at all. And the play of light reflections inside the gemstone due to the numerous facets and the proper angles gives the illusion that the entire gemstone is colour-changing, and not a minority area only.
Want to learn more about toumalines?

Start your journey to becoming a tourmaline expert with our free online course “Introduction to tourmalines”.
Learn all about tourmalines. Their fascinating history, how they form, where they come from. Learn about all the different origins of tourmalines and their treatments. Take this course as an introduction to the wonderful world of tourmalines.